In order to understand the process of reverse osmosis (RO), one should first know what is meant by osmosis.
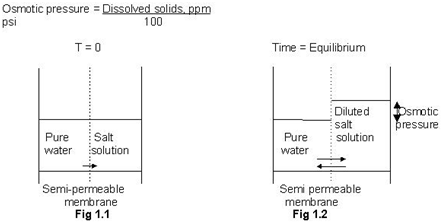
Imagine a hypothetical experiment as shown in figure 1.1. A container is divided into two compartments using a semi-permeable membrane. A semi-permeable membrane has a property of selective passage for certain substances i.e. this membrane allows only water to pass through, while salt and other components are rejected by the membrane. The left-hand compartment is filled with pure water. The right hand compartment is filled with a salt solution.
Pure water has higher chemical potential (or energy) when compared with the salt solution. Second law of thermodynamics states that the flow is always from higher energy to lower energy. Hence, the water flows from the left-hand compartment to right hand compartment. This phenomenon of transport of water across semi-permeable membrane from low salt concentration to high salt concentration is called osmosis.
Due to osmosis, the level of water in left-hand compartment falls, while the level of solution in the right hand compartment rises. Since the right hand compartment has higher head (or potential energy) now some water flows back from right hand compartment to left hand compartment. This flow in reverse direction is zero initially when both compartments have the same levels, but it increases gradually with the increase in difference between heads (or levels) of the two compartments. After some time a stage is reached when the flow due to the osmosis and the reverse flow are the same. The levels in the compartments stop changing thereafter. This condition of dynamic equilibrium is called as osmotic equilibrium. The difference between heads of the two compartments at this stage is called osmotic pressure (refer to figure 1.2 ).
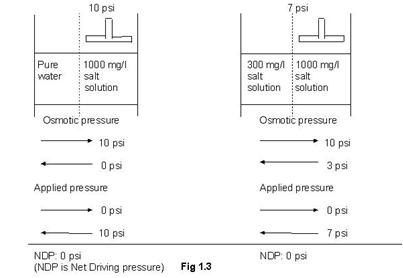
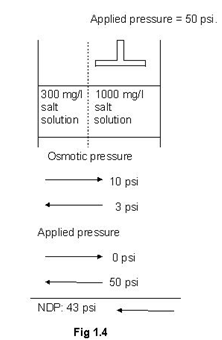
Osmotic pressure is directly proportional to the concentration of a solution. Numerically, approximate value of osmotic pressure is given as
INDION series of INDRO are field proven, highly reliable and cost effective answer to treat a wide range of brackish waters. Designed with flexibility, the series utilises state-of-the-art spiral wound reverse osmosis membranes to suit a given application. These systems can remove 90 - 98% of total dissolved salts.